
An "ingestible sensor" doesn't sound like the tastiest of snacks, but soon it might be just what the doctor ordered. A tiny microchip which activates upon contact with stomach acid has recently been given the green light by health regulatory agencies in the US and Europe. When the sensor is swallowed, an external patch picks up its signal and shoots a message over to whoever it's supposed to. The technology is aimed at tackling an issue known in the healthcare biz as compliance -- or, following instructions. Correct timing and dose are important for many drugs, and lax schedules can be responsible for treatment failures or the development of nasty drug-resistant bugs. Although the necessary trials used placebo pills, one pharmaceutical heavyweight has already bagged a license to the technology for real-world applications. If the thought of passing microchips is troubling you more than the thought of eating them, no need to worry -- the kamikaze sensors dissolve in your stomach shortly after completing their mission.
Continue reading FDA approves Proteus Digital Health's e-pills for dose monitoring
Filed under: Science
FDA approves Proteus Digital Health's e-pills for dose monitoring originally appeared on Engadget on Wed, 01 Aug 2012 17:04:00 EDT. Please see our terms for use of feeds.
Permalink |
 DVICE, Proteus Digital Health
DVICE, Proteus Digital Health |
Email this |
Comments
 30.3 million Americans have diabetes according to a 2015 CDC study. An additional 84.1 million have prediabetes, which often leads to the full disease within five years. It's important to detect diabetes early to avoid health complications like heart...
30.3 million Americans have diabetes according to a 2015 CDC study. An additional 84.1 million have prediabetes, which often leads to the full disease within five years. It's important to detect diabetes early to avoid health complications like heart...
 30.3 million Americans have diabetes according to a 2015 CDC study. An additional 84.1 million have prediabetes, which often leads to the full disease within five years. It's important to detect diabetes early to avoid health complications like heart...
30.3 million Americans have diabetes according to a 2015 CDC study. An additional 84.1 million have prediabetes, which often leads to the full disease within five years. It's important to detect diabetes early to avoid health complications like heart...
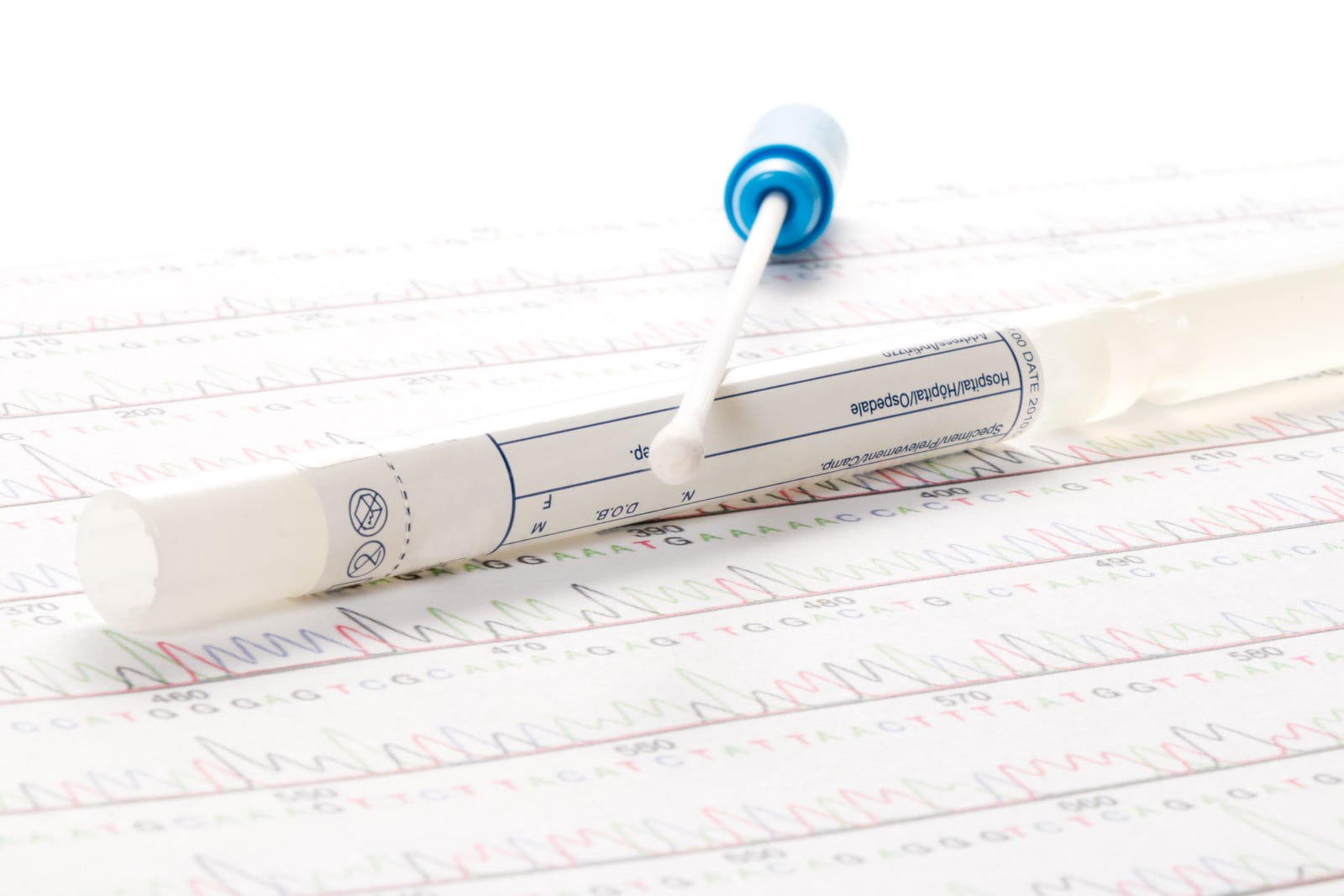 FDA Commissioner Scott Gottlieb announced new rules today regarding direct-to-consumer genetic health risk (GHR) tests and the process by which they're approved for sale. In a statement, Gottlieb explained that these sorts of tests can provide more a...
FDA Commissioner Scott Gottlieb announced new rules today regarding direct-to-consumer genetic health risk (GHR) tests and the process by which they're approved for sale. In a statement, Gottlieb explained that these sorts of tests can provide more a...
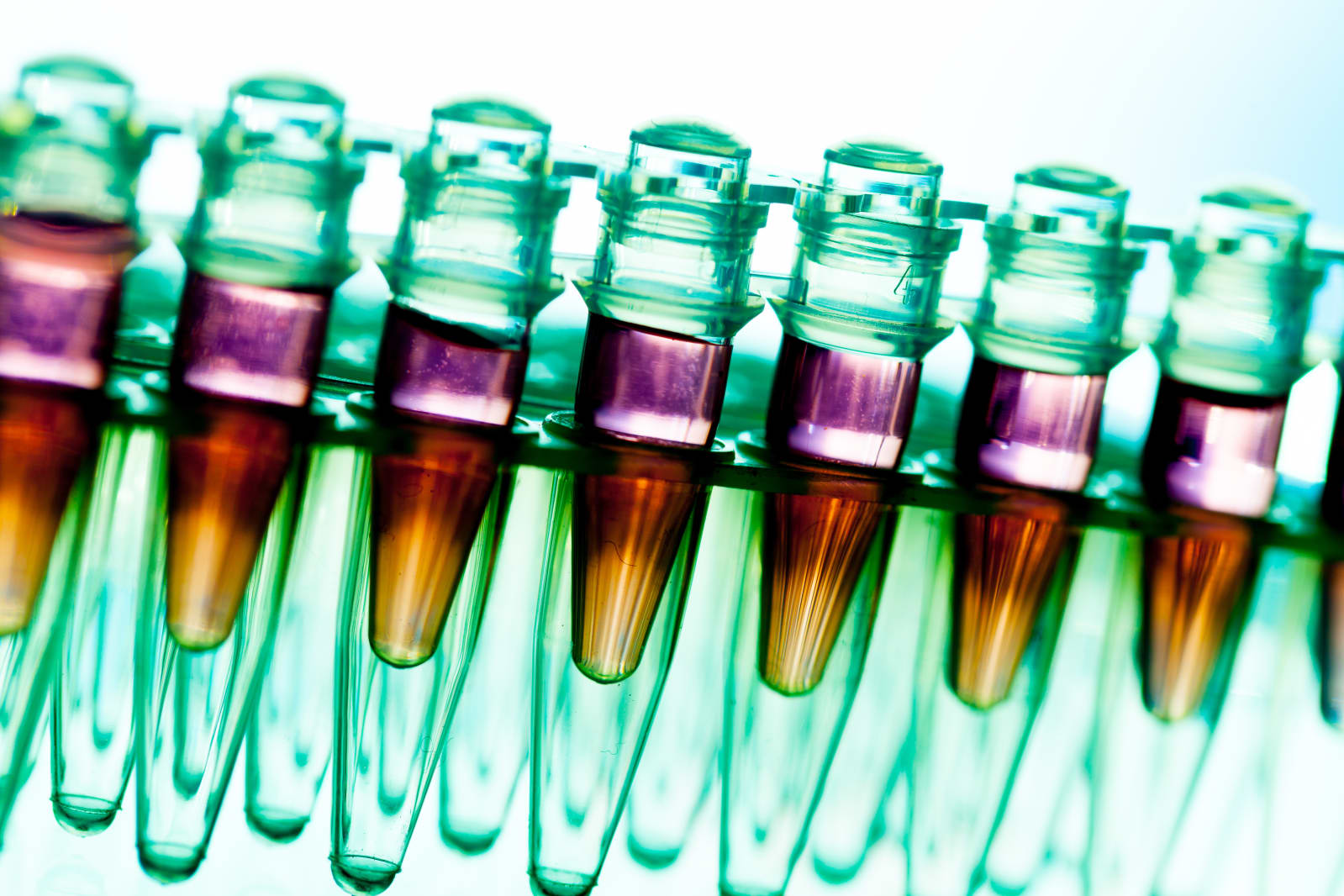 The first gene therapy treatment has been approved for use in the United States. The FDA greenlit a procedure that uses a patient's own cells to combat a particular type of leukemia, but will only permit it for children and young adults up to age 25.
The first gene therapy treatment has been approved for use in the United States. The FDA greenlit a procedure that uses a patient's own cells to combat a particular type of leukemia, but will only permit it for children and young adults up to age 25.
 The meatless Impossible Burger has been hard to track down since it debuted last August. Its parent company Impossible Foods promised that a massive new factory would put the faux beef patties in 1,000 restaurants by the end of the year, but that rol...
The meatless Impossible Burger has been hard to track down since it debuted last August. Its parent company Impossible Foods promised that a massive new factory would put the faux beef patties in 1,000 restaurants by the end of the year, but that rol...

